Please wait...
Optimizing Your Mobile Phase:
The Importance of Solvent and Buffer
| Shop Mobile Phase Modifiers | Have my Sales Representative Contact Me | Have a technical question? Ask Now |
Liquid chromatography is one of the most heavily used techniques for analyzing mixtures of compounds in solution. It can be run in analytical mode to analyze the composition of a mixture, and in preparation mode to isolate the pure components. Liquid chromatography has evolved from the initial Nobel prize research into partition chromatography by Martin and Synge almost a hundred years ago.
Partition chromatography is based on the concept that each target analyte moves between the stationary solid phase and the mobile phase. In liquid chromatography the mobile phase is composed of solvents or other liquids. The choice and composition of the mobile phase impacts the movement of analytes through the column. In the case where there are components with nearly the same retention time, very subtle factors can make a difference between seeing two components as individual peaks versus seeing just one large peak. Good resolution is seeing two narrow peaks close together; poor resolution is the opposite, one wide peak, showing that these two components were not well separated and remain mixed together. Additions of mobile phase modifiers to the solvent can fine tune the separation of these peaks.
Modifiers are pure substances that satisfy three criteria. First, modifiers don’t interfere with identifying a component of a mixture; if possible, modifiers are chosen to make it easier to identify these components as they are purified. Second, modifiers must contribute to resolving two peaks that have nearly the same retention times. Third, only a small amount of modifier is needed to achieve a given separation goal.
Modifiers can also aid in column resolution by interacting with active sites that are not bound with the stationary phase. These open sites are regions where there can be possible interaction with the analytes or attack from solvents, causing column degradation and peak broadening. They can also modify regions that are not sticky enough. The “leveling effect” of a good modifier produces more uniformity across all of the pores in stationary phase particles, as well as all spaces between particles, without causing a degradation of the particles which is called “remodeling” of the stationary phase. Fixing these types of defects in the stationary phase means no matter where the components of the mixture bind to the stationary phase, they see exactly the same environment, and the components move with the mobile phase in a consistent way, and tailing is minimized. The result of choosing the best modifier is peaks are sharp with minimal to zero overlaps.
There are various chemical properties that affect the way analytes interact with the mobile and stationary phases. These include the polarity of the solvent, as well as the miscibility of co-solvents, when a more complicated mobile phase is being used. In addition, the hydrogen bonding characteristics of the solvent and analytes have a critical effect on the ability of a column to separate each analyte into distinct peaks. For polar molecules, particularly those that can ionize, we also need to consider the pKa (acidity) of the analytes and solvent.
Polarity occurs when molecules or solutions have either a significant difference in charge, electronegativity or ionic bonds leading to high dipole moments (large differences in charge). These compounds or solvents with high dipole moments are polar, as opposed to equal sharing of bonds (covalent or polar covalent) with little to no charge and are non-polar. Polarity can be ranked using a polarity index (P’) which is a relative measure of a solvent or solution with various polar matrices. The higher the polarity index, the more polar the solvent (Table 1).

There are two types of liquid chromatography based on polarity of the phases. The first types of liquid chromatography were based on normal phase liquid chromatography (NPLC) or absorption chromatography where the mobile phase consisted of a non-polar solvent while the stationary phase was composed of polar materials such as silica. The analytes analyzed by NPLC are more hydrophobic and non-polar in nature.
Most modern liquid chromatography is based on reversed phase chromatography (RPLC) or partition chromatography in which the mobile phase is polar while the stationary phase is composed of non-polar materials such as n-octyl (C8) or n-octyldecyl (C18) hydrocarbon chains. Analytes for RPLC tend to be more hydrophilic and polar than analytes studies by NPLC.
The ability of a solvent or mobile phase to pull analytes from the stationary phase or adsorbent is called its eluent strength, elution power or eluotropic value (ε0) and is dependent upon the polarity of the chromatography phases. The eluotropic value (ε0) expresses the measurement of the solvent or mobile phases absorptive energy based on a particular substrate or stationary phase such as aluminum oxide (Table 2).

If more than one solvent is used simultaneously during a method or mobile phases are mixed, the polarity index changes. To determine the new polarity of a solution, the composition of the mixture is calculated with the known polarity indices for the solvents to obtain a new polarity index using the following equation:
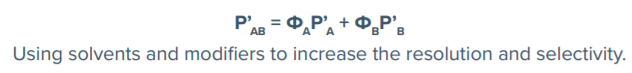
Peak resolution (the ability of a column to separate peaks in a chromatogram) is a complex interaction of forces and factors observed as adjacent peaks merging (co-eluting) to a single peak or separating (resolving) into two or more peaks. Resolution is a function of three elements including retention, selectivity and efficiency. Retention or capacity factor (k) is the time that a sample analyte resides in the stationary phase relative to the time it spends in the mobile phase. Separation or selectivity factor (α) is the ability of a chromatography system to distinguish the chemical differences between compounds. Efficiency (N or H) is a measure of theoretical plates in a chromatographic system.
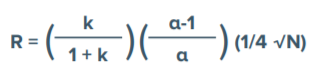
Selectivity can be changed by chemical effects to solvent through mobile phase modifiers. The chemical nature of the analytes becomes important when choosing a modifier. Modifiers have a leveling effect that can be summarized by a number called a pKa, or acidity constant. The higher the pKa, the weaker the acid. Pure acids establish an acidity in water related to their pKa; the measure of acidity parallels the pKa and is called the pH. The pH scale ranges from 0 to 14, where the low values equate to acidic compounds and high values are alkaline. Neutral for the scale of pH is 7. The pH of a solution indicates an acid or base, but not necessarily the true strength of that acid or base. To measure the strength of an acid or base, one needs to look at the pKa. Alongside acids, if one dissolves a salt of a weak acid (acid with a pKa between 3 and 7) with the acid, one has a pH-stabilizing system called a buffer. Salts of weak acids are called buffer salts; by adding buffers to mobile phase, the chromatographer can change resolution (Table 3). High levels of buffer (> 0.1 M) with extreme pH (very acidic or very basic) are discouraged because they can damage columns and increase the viscosity of the mobile phase, thereby increasing pressure in the system. For basic analytes or mixed sample types, mobile phase pH near neutral increases retention, while acidic samples are aided by acidic mobile phase.
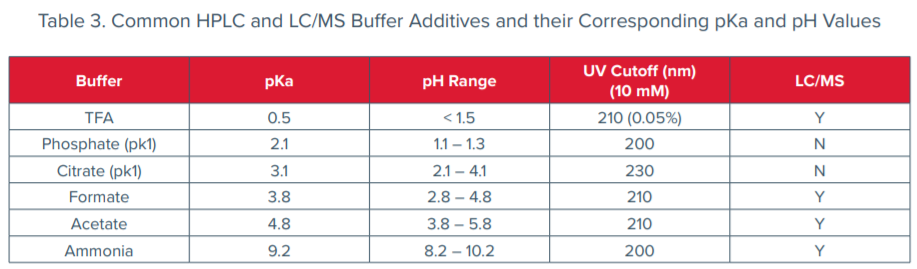
Differences between target pKa (or pKb) and mobile phase pH and pKa are of even more importance in LC/MS where ionization is critical to detection. Keeping mobile phase (and buffers) pH and pKa similar to the target improves resolution because it moves the ionizable target to a more neutral state, but for LC/MS to be effective, the compound needs ionic character or have the ability to be easily ionized. Therefore, the rule of thumb for pH, pKa and mobile phase is to aim for mobile phase pH 1-2 units from the target analytes pKa within the specification range of the column.
It is important to remember that not all buffers are appropriate for all applications. Salts can precipitate out of solution as mobile phase composition changes. Salts are also problematic for LC/MS analysis since they can inhibit MS detection. If solids are dissolved into mobile phase, it is important to remember to filter the mobile phase and, in some cases, the mobile phase may need to be heated to dissolve solids completely, in which case the column of stationary phase may also have to be kept warm. The totality of factors that enable a good separation of a mixture is called the “method” of the purification. It is important to understand that the first changes during method development often revolve around changes to the chemistry and composition of the mobile phase. There are a lot of different choices which can be made regarding solvent, polarity, modifiers, and chemical conditions which can influence resolution. The best approach is to make changes one at a time and work in a slow, steady pace to optimize all of the parameters of the mobile phase, stationary phase and system to achieve the best method for your analysis.
| Download the complete technical note |